Boosting growth hormone without injections
November 28th, 2016Boosting growth hormone without injections
Updating the GHRPs and Sermorelin by Richard Walker, M.D.
Ed. – Eagle eyed readers may recall Dr. Walker’s other excellent articles regarding GHRPs and GHRH in issue 3, 2014 and issue 3, 2013 of the Aging Matters™ magazines, (note, they can be downloaded from the IAS website). In them he described how bolus injections of recombinant growth hormone (rhGH) do not reproduce bioidentical patterns of GH release from the pituitary, whereas application of GHRPs and sermorelin do naturally accentuate the pulsate releases of GH that occur throughout the day. His avocation is that long-term administration of injections of GH could lead to downregulation of the pituitary’s own production, which would not be desirable. In this article, Dr. Walker continues to assert that the peptides are a more effective long-term therapy to boost GH levels and that they are effective orally (unlike GH itself). His conclusion, also confirmed to me via personal communication, is that GHRPs can be used concurrently alongside sermorelin, but that just like any hormone treatment they should not be used continuously, (i.e. there should be frequent breaks) and that they should be used judiciously and sensibly etc.
Sermorelin is a truncated analog of growth hormone releasing hormone (GHRH) and the GH releasing peptides, (GHRP’s) like GHRP2 and GHRP6 are various peptidyl analogs of ghrelin. These substances have been used for over two decades in clinical and research protocols intended to oppose maladaptive, age-associated changes in body composition.
Specifically, these substances are secretagogues that enhance pituitary GH activity so as to sustain or increase lean body mass, while decreasing total and visceral body fat.
However, despite widespread use of these molecules in age management, some practitioners still lack a thorough understanding of their developmental histories, structural peculiarities and functional relationships. For example, recently the question was raised whether GHRP2 and/or GHRP6 are structurally unrelated analogs of GHRH or simply bioactive fragments of the 44 amino acid neuropeptide. In fact, they are neither. The GHRP’s are not related structurally or functionally to GHRH. Their structures evolved from repeated chemical modifications of met-enkephalin, an opioid that acts as a growth factor in neural and non-neural cells and tissues, in addition to being a neurotransmitter and neuromodulator (1). However, despite their origins, GHRP’s are not opioids. Functionally, they display the properties of ghrelin, a 28 amino acid peptide found primarily in the stomach that was discovered by Kojima and colleagues in 1999 (2).
Ghrelin is also found in the brain where it is thought to modulate the activity of GHRH-releasing neurons in conjunction with dopamine cells (2, 3, 4). In contrast, sermorelin is a bioactive analog of GHRH that is represented by the first 29 amino acids in the 44 amino acid sequence of the intrinsic GH releasing factor. Consistent with their roles in controlling pituitary activity, both categories of GH secretagogues have their own distinct, saturable receptors on somatotrophs that have separate, complementary functions in regulating GH production and secretion (5). Not only that, but when analyzing responsiveness to GHRH and/or GHRP by double-reverse hemolytic plaque assay, the presence of at least three functionally distinct somatotroph subpopulations were identified (6).
Until the discovery of GHRPs, control of pituitary GH production and secretion was thought to be regulated by the stimulatory and inhibitory influences of GHRH and somatostatin, respectively (7). In turn, the activity of cells responsible for production and release of these neuropeptides were known to be regulated by brain catecholamines. Specifically, the neurotransmitter systems responsible for stimulating GHRH and/or inhibiting somatostatin are adrenergic catecholamines and dopamine acting via α2- receptors and D1 or D2 receptors, respectively.
Excitatory amino acids acting via N-methyl-D-aspartate (NMDA) and non-NMDA receptors also similarly influence GHRH and SRIF (somatotropin release inhibiting factor) see figure 1.
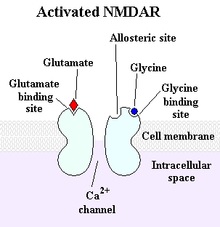
Figure 1: The NMDA receptor: a type of glutamate receptor that participates in excitatory neurotransmission has some influence over GHRH and SRIF.
Anecdotally, this effect accounts to some extent for the ability of certain amino acids such as glutamate, glycine, D-serine and aspartate to act as weak, non-specific GH secretagogues. In contrast to the α-adrenergic neurotransmitters that enhance pituitary GH activity by stimulating and inhibiting GHRH and SRIF, respectively, β-adrenergic systems have the opposite effect, thereby providing CNS control over GH homeostasis.
Before synthesis of GHRPs and subsequently the discovery of ghrelin, it was believed that the regulatory system for GHRH and SRIF previously described was complete. However, ghrelin/GHRPs were subsequently found to also have a significant physiological role in the regulatory relationship between GHRH and somatostatin. This fact was initially implied by the finding that GHRP profoundly stimulated GH release when administered in vivo but not in vitro, where the GH response was very weak. However, when hypothalamic fragments were incubated with pituitary cells, the in vitro response was augmented. Furthermore, when GHRH was incubated with pituitary cells plus GHRP, GH release exceeded even that produced by GHRH alone. In fact, the effect was synergistic rather than additive (see figure 2).
This finding suggested that GHRP and GHRH release GH by different and complementary mechanisms whereby GHRP is dependent on endogenous GHRH, which in turn plays a permissive role in releasing GH from the pituitary (8).
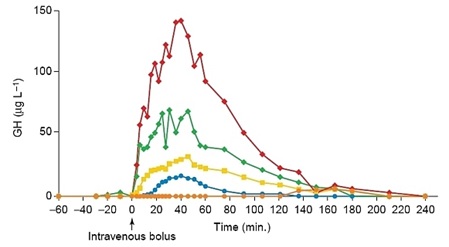
Figure 2: Comparative GH responses in individual human subjects. GH responses together with areas-under-the-curve, (µgL-1 after 4-hours). Placebo, (orange, 540); 0.1 µg kg-1 GHRP (blue, 916); 1 µg kg-1 GHRP, (green, 5319); 1 µg kg-1 GHRH, (yellow, 2590); 0.1 µg kg-1 GHRP plus 1 µg kg-1 GHRH (red, 10,065). From Bowers et al., 1990 J Clin Endocrinol Metab, 70:975-982
Note in Figure 2. that when GHRP is administered in vivo at the same dose as GHRH, it releases more GH than the primary neuropeptide, demonstrating the dependence of GHRP upon GHRH for activity. But, GHRP does not passively participate in enhancing GHRH efficacy. It also stimulates hypothalamic release of the neuropeptide. Evidence for this effect derives from several facts., GHRPs have specific receptors in the brain. and their natural analog, ghrelin also exists in the brain. Cells containing ghrelin were visualized specifically within the arcuate nucleus wherein resides GHRH cell bodies (Figures 3, 9, 10).
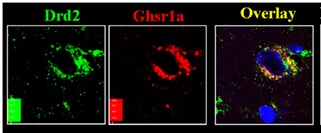
Figure 3: Ghrelin-producing neurons and binding sites in the arcuate nucleus indicate a central effect for GHRPs in modulating GHRH release and thus enhancing natural functions of GHRH neurons. Source: Kern et al., Front Endocrinol 2014. 5: 1-8.
In relation to enhancement of GHRH neuronal functions, the ghrelin-producing neurons were found in association with dopamine signaling (11). The relevance of this finding derives from the fact that dopamine provides stimulatory control of GHRH neurons as previously mentioned. That these structural relationships are functional was demonstrated by the fact that GHRP’s activate a subset of neurosecretory cells in the arcuate nucleus, of which a significant portion are GHRH- synthesizing (12, 13).
This functional relationship has been supported by measurements of GHRH in the hypothalamo-pituitary portal circulation following activation (14, 15). Finally, the synergistic effect of GHRP upon GHRH results also in part, from its ability to suppress somatostatin release from the hypothalamus and somatostatin effectiveness on the pituitary (16).
The GH-releasing activity of ghrelin/GHRP in vivo is dependent upon endogenous GHRH and GH secretion, and is not maintained in the absence of GHRH. The primary feed-forward regulator of GH secretion is GHRH. Thus, ghrelin/GHRP is considered to be a facilitator or modulator of GHRH in the regulation of GH secretion (a diagrammatic representation of these relationships is provided in Figure 4).
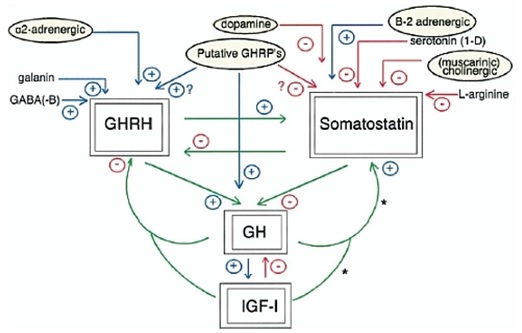
Figure 4: Stimulatory and inhibitory roles of neuropeptides and neurotransmitters that modulate GH secretion via GHRH or SRIF, or act directly on the pituitary gland. Ghrelin/GHRP is central in facilitating GH secretion by stimulating GHRH, inhibiting SRIF and acting directly on the pituitary. Source Muller et al. Physiol Rev 1999; 79:511-607 (130)
Clinical considerations
Utilization of GHRH and ghrelin analogs to increase GH secretion- as part of an age management paradigm- requires more than an understanding of the facilitating effects of sermorelin and GHRP. Under the dynamics of natural conditions these are physiological relationships acting in balance to sustain neuroendocrine homeostasis. When the injected secretagogues are administered, they act as pharmacological agents that no longer observe such balance. On the contrary, they tend to disrupt internal order depending upon the dose, frequency and timing of their administration.
By virtue of parenteral administration, the injected secretagogues are presented as boluses that are generally larger than those which the body experiences under physiological circumstances. Hence, considerations of feedback effects and receptor desensitization must be carefully considered. Long feedback loops with easily recognizable structural impacts on the body strikingly demonstrate the potential of hormone administration to accomplish a desired clinical outcome while bringing with it an undesirable side effect. For example, anabolic steroids such as testosterone are very effective in increasing muscle mass and reducing body fat. However, they also shrink testicular size and volume due to profound negative feedback on pituitary gonadotropin secretion.
Similarly, the use of recombinant human growth hormone (hGH) essentially shuts down pituitary somatotroph function, depending upon the dose administered. In effect, the desired pharmacological effect of improving body composition during aging is accompanied by the paradoxical side effect of accelerating pituitary functional decline that is the result of aging in the first place!
As a result of such negative effects on total body neuroendocrine homeostasis, it is advisable to select an intervention that is sufficiently high on the feedback loop to avoid negatively impacting as much of it as possible. However, no matter how much care is taken it is inevitable that some part of the system will be “shut down.” So if possible, only the shortest feedback loop should be affected in hormone replacement therapy. As shown in Figure 5, the GH neuroendocrine axis involves many levels of feedback control including long, short and ultra-short loops.
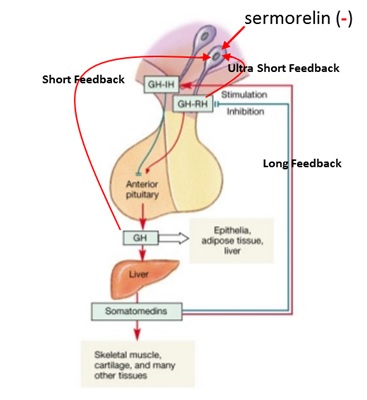
Figure 5: Long feedback involves the somatomedins (IGF-1 et al.) having negative and positive feedback on GHRH and SRIF, respectively. Short feedback involves GH having negative and positive feedback on GHRH and SRIF, respectively and ultra-short feedback involves GH and GHRH having negative feedback on somatotrophs and GHRH-producing neurons. Thus, while “external” factors can modulate the activity of GHRH neurons, GHRH can also influence its own production and secretion.
Missing from Figure 5 is Ghrelin/GHRP, because its effect is contrary to the rest. Since GHRP stimulates GHRH neurons and inhibits SRIF neurons, it can “feed forward” so as to somewhat override negative feedback factors upon GHRH neurons. Also, while the GHRH analog sermorelin avoids some of the short negative feedback resulting from clinical use of rhGH, chronic use of the smaller peptide is hampered by a rapid desensitization of its intended response. Such desensitization of the GHRH receptor (GHRH-R) after long and short-term exposure to its agonist has been described in vivo and in vitro (17-19).
The combined effect of ultrashort loop feedback and GHRH-R desensitization can explain a common complaint of practitioners who use serum IGF-1 levels as a primary marker of sermorelin efficacy. The oft-repeated complaint is that unlike after rhGH administration, sermorelin causes IGF-1 levels to increase initially but then after a few months to decline. In contrast, IGF-1 levels remain elevated after GHRP (Figure 6).
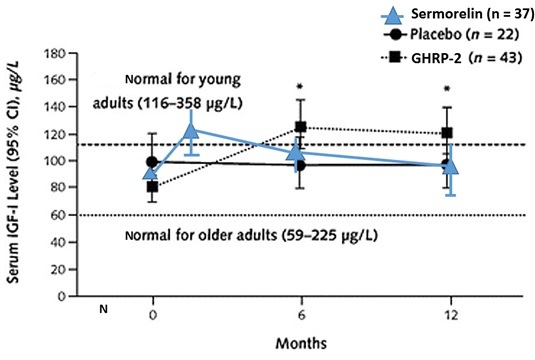
Figure 6: Differential effects on sermorelin and GHRH on serum IGF-1 levels.
Chronic administration of sermorelin, but not GHRP, is accompanied by an apparent loss of sermorelin efficacy. This is due to a combination of factors, including ultrashort loop feedback resulting in depletion of endogenous GHRH along with desensitization of GHRH receptors and reduction of GHRH-R messenger RNA accumulation (20). Both of these effects are caused directly by repeated daily injections of pharmacological doses of sermorelin, since negative feedback sites, SRIF neurons and GHRH-R mRNA “see” sermorelin as excessive amounts of GHRH and thus, shut down production of the endogenous stimulatory neuropeptide and its receptors.
This effect takes several weeks, and thus allows the additive effect of sermorelin and endogenous GHRH to occur. This results in an initially significant increase in circulating hGH and IGF-1. However, after continued daily administration of sermorelin, its negative effects on brain and pituitary GHRH receptors reduces the amount of hGH and IGF-1 that results from the same dose of analog that previously produced a robust effect (Figure 7).
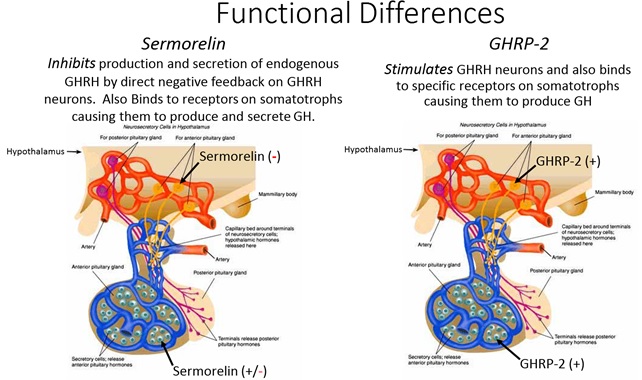
Figure 7: Differential effects of sermorelin and GHRP upon endogenous GHRH in brain and pituitary. Even though daily injections of sermorelin are capable of shutting GHRH production in neurons and synthesis of GHRH receptors in brain and pituitary, these effects are fully manifest after a period of time. Thus, initially sermorelin results in an additive effect on endogenous GHRH receptors, but after several weeks or months, the absence of natural GHRH and desensitized GHRH receptors blunt the initial response. In contrast, GHRP stimulates GHRH production and sustains its receptors (20, 21).
As seen in Figure 7, GHRP stimulates GHRH neurons and hence sustains its receptors by direct effect and also due to association of its receptors with those of dopamine to form GHSR1a:DRD2 heteromers (11). Furthermore, GHRP does not desensitize GHRH receptors on somatotrophs while at the same time, enhancing efficacy of endogenous GHRH through synergy. These functional differences have clinical significance when considering interventions intended to restore somatotroph functions that fail during aging, primarily because of progressive deficiency in endogenous GHRH and SRIF dominance (22).
GHRP not only stimulates GHRH and inhibits SRIF, it also enhances GHRH potency in somatotrophs by activating specific receptors that act to amplify second messenger systems leading to increased production and secretion of GH (Fig 8). Recrudescence is facilitated by GHRP, because it recruits separate somatotrophs from the 3 subpopulations (6).
Somatotrophs Have Dual GH Secretagogue Receptors
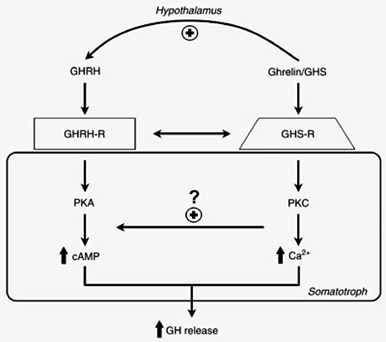
Figure 8: Separate somatotroph receptors for GHRH/sermorelin and ghrelin/GHRP underlie their synergy. Even in the presence of relatively low concentrations of GHRH, its effect on second messengers within the somatotroph is amplified by the activation of GHRP receptors on the same cell and in subpopulations of other somatotrophs (5, 6). Source: M.J. Lengyel. Braz J Med Biol Res 2006; 39: 1003-1011
It is also important to note that even when GHRP is administered to older persons, GH secretion is greater even than that resulting from administration of GHRH or its analog, sermorelin, so long as some level of endogenous GHRH is present (Figure 9).
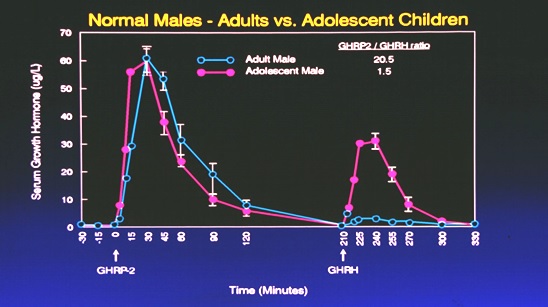
Figure 9: Comparative responses to i.v. administration of GHRH or GHRP in adolescent and adult subjects. Subjects were first administered GHRP so as to avoid possible synergy with GHRH. Accordingly, GHRH was administered separately over three hours following the first dose of GHRP. Source: Walker RF, Bercu BB JAAM 1:219-225, 1998
As seen in Figure 9, the response to GHRP in adolescent and adult subjects was comparable even though endogenous GHRH declines with advancing age. Thus, the data suggest that the synergistic response to GHRP is not dose related to GHRH, but rather just the presence of some is sufficient to realize the full stimulatory potential of GHRP. On the other hand, the additive effect of exogenous GHRH with endogenous GHRH reflects the age-related decline in the latter neuropeptide with advancing age. When GHRH was administered to adolescents, their peak response was over 10 times greater than that seen in adults. These data suggest that GHRP is a better choice for opposing pituitary dysfunction during aging than is sermorelin.
A paradoxical effect of GHRP to increase body fat is sometimes noted when sermorelin which has been used for prolonged periods is discontinued and immediately replaced by the ghrelin analog. As has been demonstrated repeatedly by many different researchers, GHRP increases lean body mass and reduces total body fat, presumably as the result of its ability to activate the GH neuroendocrine system. However, it is important to understand that ghrelin/GHRP is also orexigenic, and thus has the ability to increase appetite and body weight. Generally, this effect is not observed at low doses of GHRP that stimulate the GH neuroendocrine axis. However, it is important to remember that GHRP does not release GH in the absence of GHRH (8). Such circumstances may be permissive to the orexigenic effect of GHRP which otherwise would be blocked by its low-dose, GH secretagogue activity. An animal model for this paradoxical effect is seen in rats with mutated GHRH genes (23). Because they produce no endogenous GHRH, such mutated rats gain fat and body weight when given GHRP. Since as previously discussed, chronic administration of sermorelin suppresses GHRH production of neuropepetide and synthesis of its specific receptor thereby essentially eliminating the endogenous secretagogue. Thus, if GHRP is administered immediately after cessation of sermorelin therapy without allowing sufficient time for recrudescence of the intrinsic GHRH system, then only its orexigenic effects will be manifest and fat, rather than muscle, will increase. Accordingly, a “recovery” period of at least one or two months is recommended before switching from sermorelin to GHRP for GH secretagogue, antiaging therapy.
Alternatively, GHRP may be given in conjunction with sermorelin so as to sustain the beneficial effects of secretagogue therapy on body composition. However, the practitioner should be aware that such “combination therapy” overrides and eliminates the naturally occurring temporal relationships between the brain and pituitary governing GH secretory circadian rhythms. In contrast, GHRP alone may not cause significant perturbation of such physiological rhythms because it facilities, rather than initiates, the process of GH neuroendocrine activity. Of course, GHRP may be used in any patient who is not taking sermorelin to achieve full benefit of its influence over the GH neuroendocrine axis.
Another issue that is of relevance to clinical use of GH secretagogues is their toxicity profile. Recently this question arose following the finding that Foreo® (teriparatide), a truncated analog of naturally-occurring parathyroid hormone (PTH) causes osteosarcoma in animals, and possibly in humans as well. Like sermorelin, which contains 29 of the 44 amino acids that make up GHRH, teriparatide contains 34 of the 84 amino acids composing PTH. Because of this structural peculiarity of the two endocrine analogs, the question of whether fragments of naturally occurring peptides are potentially carcinogenic was raised. That possibility is rather doubtful, since it is more likely that the specific impact of the peptide analog upon its target tissue is more significant than the fact that it is a fragment of the naturally occurring molecule. For example, it has been reported by the manufacturer of Forteo that “intermittent spikes of PTH, such as given by daily injection, will cause more increase in bone formation than in bone resorption.”(24). This imbalance in bone homeostasis apparently leads to development of osteosarcoma due to abnormal proliferation of osteoblasts”.
It should be noted that unlike sermorelin or GHRP that are subject to any number of feedback controls, which prevent excessive stimulation of pituitary cells, teriparatide directly stimulates bone cells and is not under feedback control. Accordingly, the persistent and unimpeded stimulation favoring osteoblasts over osteoclasts directly disrupts bone homeostasis. This effect probably has little to do with the fact that teriparatide is a fragment of PTH and more to do with differences in its pharmacology from the native hormone (25). This possibility is totally different from the cases of sermorelin and GHRP since both are analogs of GHRH and ghrelin, respectively, and bind to the same receptors in brain and pituitary as the natural peptides. Thus, it is unlikely that hyperstimulation of any cell population with the potential to cause hyperplasia (and worse) is not likely. In fact, unlike teriparatide, there is no evidence that sermorelin, GHRP or even hGH causes cancer de novo (26). However, because GH is mitogenic, the secretagogues and hGH are contraindicated in extant cancer, but they can be used in periods of remission.
Furthermore sermorelin and GHRP have been used in animal studies and human clinical trials for decades without any reports of serious adverse events associated with their use. A comparison of mild side effects with low incidence that were reported for sermorelin and GHRP is presented in Table 1.
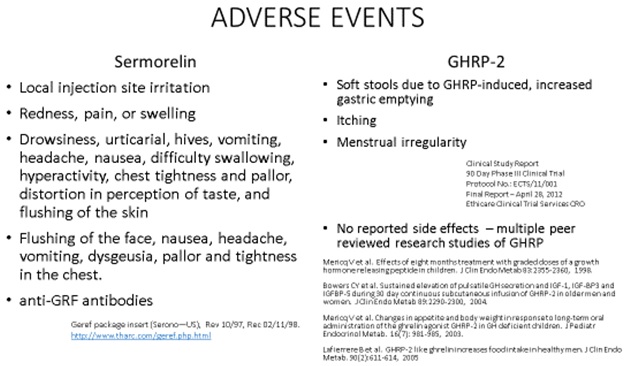
Table 1: A comparison of adverse events reported in studies with sermorelin and GHRP. All were Grade 1 Mild AE which is the lowest level AE with Grade 5 being the most serious.
The delivery systems
Traditionally, peptides are administered by subcutaneous or intravenous injection. While efficient for drug administration, the delivery system has the disadvantage of presenting a secretagogue as an instantaneous “pulse” rather than a slower, more persistent and longer-lasting presence in the bloodstream.
Infusions of GHRP in humans enhanced pulsatile GH secretion and increased plasma IGF-I concentrations without significant adverse effects (27). These findings were in contrast to secretory responses to bolus administration, suggesting that a slower more continuous presence of GHRP is more advantageous than brief, daily pulsatile exposure to the secretagogue in restoring GH neuroendocrine homeostasis. Obviously, it is impractical to even consider infusion as a strategy for pituitary recrudescence during aging. However, oral administration which provides a slower, more constant means of delivery might be more clinically advantageous and effective for that reason alone.
To this point, we recognized that peptidyl GHRPs are composed in large part of D-amino acid isomers which, unlike the l-isomers, are resistant to endopeptidase attack in the gut (28). As a result, they are orally bioavailable and suitable for use in oral dosage forms (29). Accordingly, we created novel oral formulae for use in opposing GH neuroendocrine decline and its sequelae during aging. In addition to providing a more prolonged presentation of secretagogue to the brain and pituitary, another advantage of such formulations is that complementary ingredients can be included in the single formulation. Some of these were added to enhance dopamine and other neurotransmitter activity as well as to promote pituitary sensitivity and to directly increase skeletal muscle development. GHRP2-Pro™ or Release-Pro™ are formulations that can be used in lieu of sermorelin, but will still provide all the benefits of enhanced GHRH activity as previously described. All ingredients have been subjected to extensive testing and clinical applications with a high safety profile. In addition to such clinical trials, there is a literature of their use in humans for up to a year or more, including children, without a single report of toxicity. In preclinical safety testing they have a clean record in acute (LD50 testing), repeated study (90 day dosing/histopathologic examination of all tissues at necropsy), and genotoxicity.
GHRP2-Pro™ can be used in conjunction with sermorelin to promote even greater synergy between the GHRH and ghrelin systems. But if such combination therapy is considered, the physician should advise the patient to discontinue sermorelin and any source of GHRP including GHRP2-Pro™ until adequate recovery of endogenous GHRH has occurred. Only thereafter should GHRP be employed alone as an effective GH secretagogue because of its absolute dependence upon endogenous GHRH for efficacy, and to avoid its inherent ghrelin-like orexigenic effects.
Conclusion
Based upon the data presented above, it is concluded that sermorelin and GHRP are safe and effective for use GH replacement age-management protocols. However, because of their relative positions in the hierarchy of the hGH neuroendocrine axis, GHRP offers certain advantages over sermorelin for long-term therapy. These advantages of GHRP are summarized simply by noting that because the body “sees” sermorelin as GHRH, then normal physiological processes are activated so as to restrict the amount of stimulation resulting from administration of pharmacological doses of the GHRH analog. In other words, ultra-short loop feedback, receptor desensitization and stimulation of SRIF production and secretion occur so as to maintain homeostasis at levels consistent with age within the axis.
On the other hand, GHRP administration creates an enhanced “feed forward” situation in which activation of GHRH neurons increases, essentially from outside the main axis, and thus, shifts activity relationships within, upward. As a result, negative feedback, receptor desensitization and SRIF secretion are reduced relative to the increased GHRP influence causing in turn, greater recrudescence of somatotroph function with increased hGH and IGF-1 production.
An additional advantage of GHRP over sermorelin is that it is orally bioavailable. As previously noted, slower and more extended exposure of the hGH neuroendocrine axis to GHRP amplifies not only its quantitative activity but also sustains its natural rhythms which are disrupted by bolus injections of sermorelin. Furthermore, because of its oral bioavailability, GHRP may be incorporated in formulations such as GHRP2-Pro™ with complementary ingredients to promote a more holistic approach to therapy.
Thus, in contrast to sermorelin, more effective, beneficial and physiological outcomes in sustaining hGH neuroendocrine health and vitality can be expected from GHRP protocols such as GHRP2-Pro™.
References
- Bowers C. Unnatural Growth Hormone-Releasing Peptide Begets Natural Ghrelin. The Journal of Clinical Endocrinology & Metabolism Vol. 86, No. 4
- Kojima M1, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. 1999 Dec 9;402(6762):656-60.
- Ferrini F et al. Ghrelin in central neurons. Current Neuropharm 2009. 7:37-49
- Sato T, Fukue Y, Teranishi H, Yoshida Y, Kojima M. Molecular forms of hypothalamic ghrelin and its regulation by fasting and 2-deoxy-d-glucose administration. Endocrinology. 2005 Jun; 146(6):2510-6.
- Lengyel MJ. Novel mechanisms of growth hormone regulation: growth hormone releasing peptides and ghrelin. Braz J Med Bio Res 2006;29:1003-1011
- Mitani M, Kaji H, et al. Growth hormone releasing peptide and GH releasing hormone stimulate GH release from subpopulations of somatotrophs. J Neuroendcrinol 1996. 8(11):825-830.
- Fukata, J., D.-J. Diamond & J.-B. Martin: Effects of rat growth hormone (rGH)-releasing factor and somatostatin on the release and synthesis of GH in dispersed pituitary cells. Endocrinology 1985, 117, 457–467.
- Bowers CY, GHRP Historical Perspective/Basic and Clinical, Human Growth Hormone Research and Clinical Practice. Smith RG, Thorner MO (Eds) Humana Press Inc., Totowa, NJ, 2000, pp 17-43
- Muccioli G, et al. Specific receptors for synthetic GH secretagogues in the human brain and pituitary gland. Journal of Endocrinology (1998) 157, 99–106
- Kageyama H et al. Visualization of ghrelin-producing neurons in the hypothalamic arcuate nucleus using ghrelin-EGFP transgenic mice. Regul Pept. 2008 Jan 10;145(1-3):116-21. Epub 2007 Sep 25.
- Kern A, Grande C, Smith Apo-ghrelin receptor (apo-GHSR1a) regulates dopamine signaling in the brain. Front Endocrinol 2014. 5: 1-8. doi: 10.3389/fendo.2014.00129
- Dickson SL, et al. Retrogradely labelled neurosecretory neurons of the rat hypothalamic arcuate nucleus express fos protein following systemic injection of growth hormone (GH)-releasing peptide (GHRP-6). J Endocrinol 1997, 151:323–331
- Dickson SL, Luckman SD 1997 Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH releasing peptide-6. Endocrinology 138:771–777
- Guillaume V, et al. Growth hormone (GH)-releasing hormone secretion is stimulated by a new GH-releasing hexapeptide in sheep. Endocrinology 1994, 135:1073–1076
- Fletcher TP, Thomas GB, Clarke IJ Growth hormone-releasing hormone and somatostatin concentrations in the hypophysial portal blood of conscious sheep during the infusion of growth hormone-releasing peptide-6. Domest Anim Endocrinol 1996, 13:251–258.
- Conley L.K et al. Mechanism of Action of Hexarelin and GHRP-6: Analysis of the Involvement of GHRH and Somatostatin in the Rat. Neuroendocrinology 1995;61:44-50.
- Hansen BS et al. The Growth Hormone-Releasing Hormone Receptor: Desensitisation Following Short-Term Agonist Exposure. Pharmacology & Toxicology 2001, 88, 81–88.
- Bilezikjian, L.-M., Seifert H, Vale W: Desensitization to growth hormone-releasing factor (GRF) is associated with downregulation of GRF-binding sites. Endocrinology 1986, 118, 2045–2052.
- Vance, M. et al. Dual effects of growth hormone (GH)-releasing hormone infusion in normal men: somatotroph desensitization and increase in releasable GH. Clin. Endocrinol. Metab. 1986, 62, 591–594.
- Aleppo G, et al. Homologous down-regulation of growth hormone-releasing hormone receptor messenger ribonucleic acid levels. 1997 Mar;138(3):1058-65.
- Veeraragavan K, Sethumadhavan K, Bowers CY. Growth hormone-releasing peptide (GHRP) binding to porcine anterior pituitary and hypothalamic membranes. Life Sci. 1992;50(16):1149-55.
- Ge F et al. Relationship between growth hormone-releasing hormone and somatostatin in the rat: effects of age and sex on content and in-vitro release from hypothalamic explants. J Endocrinol. 1989 Oct;123(1):53-8.
- Alba M et al.Effects of long-term treatment with growth hormone-releasing peptide-2 in the GHRH knockout mouse. Am J Physiol Endocrinol Metab 2005, 289: E762–E767.
- Medication Guide. HIGHLIGHTS OF PRESCRIBING INFORMATION FORTEO® (for-TAY-o) teriparatide (rDNA origin) injection Lilly USA, LLC, Indianapolis, IN 46285, USA Copyright © 2002, 2013, Eli Lilly and Company,
- Anthony B. Hodsman et al. Parathyroid Hormone and Teriparatide for the Treatment of Osteoporosis: A Review of the Evidence and Suggested Guidelines for Its Use Endocrine Reviews, 2005, 26( 5): 688–703
- Jenkins PJ, Mukherjee A, somatotropin release inhibiting factor Clin Endocrinol (Oxf). 2006 Feb;64(2):115-21.
- Huhn WC et al. Twenty-four-hour growth hormone (GH)-releasing peptide (GHRP) infusion enhances pulsatile GH secretion and specifically attenuates the response to a subsequent GHRP bolus. J Clin Endocrinol Metab. 1993 May;76(5):1202-8.
- Fominaya et al. Strategies to stabilize cell penetrating peptides for in vivo applications. Therapeutic Delivery. 2015. 6(10): 1171-1194 , DOI 10.4155/tde.15.51
- Walker et al. Oral activity of the growth hormone releasing peptide His-D-Trp-Ala-Trp-D-Phe-Lys-NH2 in rats, dogs and monkeys. Life Sciences 1990, 47:29-36.