
Synergy- the story of GHRH and GHRPs
November 26th, 2016Synergy- the story of GHRH and GHRPs
Background – Age-associated hGH insufficiency: It is well known that serum titers of human growth hormone (hGH) decline during aging and that replacement of the hormone opposes many maladaptive changes in the body associated with senescence (Rudman et al., 1990). Originally, the most obvious and logical approach to therapy was administration of recombinant hGH. However, while effective in opposing undesirable changes in body composition and some other aspects of aging, hGH administration also brought with it certain undesirable side effects, abuse potential and regulatory liabilities (Walker, 2006). Furthermore, its administration as a bolus injection prevented simulation of the physiological pattern of secretion which is an important aspect of the hGH functional profile. Endogenous hGH is released in episodes that are essential for normal responses by its target tissues. These pulses attenuate with aging (Fig1).
Fig. 1: Growth hormone is released from the pituitary gland in “pulses” resulting from an interplay of stimulatory (growth hormone releasing hormone) and inhibitory (somatostatin) signals from the brain. The pulses decline during aging, especially with loss of the nighttime surge of hormone release during slow wave sleep. The age-related decrease in mean serum hGH is primarily due to reduced pulse amplitude
In contrast, exogenous hGH administration produces constant, non-physiological exposure that can result in tissue hyperstimulation, organ hypertrophy, hyperplasia and tachyphylaxis (Fig. 2).

Fig 2. Subcutaneous injection of recombinant hGH results in elevated levels of serum hormone that result in constant non-physiological stimulation of peripheral receptors. Intravenous administration has the same effect except that the hormone is cleared from the body more rapidly. Constant exposure to hGH resulting from pharmacological administration increases risk for side effects.
Paradoxically, exogenous hGH can also exacerbate aging in certain tissues such as the pituitary gland and brain because it presents strong negative feedback upon higher levels of the hGH neuroendocrine axis that “shut down” cellular functions causing disuse atrophy. Specifically this negative effect can be seen in those cells that secrete growth hormone releasing hormone (GHRH) from the hypothalamus, and those that produce endogenous hGH in the pituitary gland called somatotrophs.
GHRH/Sermorlin: The use of secretagogues is currently favored as a physiological approach to age-management medicine. The evolutionary history of molecules currently available for hGH replacement therapy is quite interesting because the discovery of one was intentionally directed, while that of the other was totally serendipitous. Search for the structure of the first secretagogue, GHRH, identified two forms including GHRH-(1-44)-NH2 and GHRH-(1-40)-OH that were extracted and fractionated from human hypothalamic tissues (Ling et al, 1984). Subsequently, structure/activity relationships showed that the same amino (NH2)-terminal, 29 residues of both molecules is involved in binding to the GHRH receptor, is responsible for biological activity and that the intracellular second messenger affecting molecular events initiated by GHRH is cyclic adenosylmonophosphate (cAMP). Based upon this information, the synthetic analog, growth hormone releasing factor 1-29-NH2 (GRF1-29NH2) or sermorelin was developed for clinical application. In vitro studies demonstrated that sermorelin binds to saturable GHRH receptors on somatotrophs, initiates transcription of the hGH gene, and supports hGH production and secretion by an intracellular mechanism involving cAMP. These findings indicated that GHRH and its analog sermorelin provide the same, primary stimulatory signal controlling hGH secretory dynamics in the pituitary gland.
GHRP/GHS: In contrast to GHRH/sermorelin another hGH secretagogue was identified using a novel method of discovery that was unrelated to extracting and fractionating tissues. Instead, the molecular structures of known neuroactive peptides were chemically modified based upon the assumption that some might be related to GHRH. Presumably, modest molecular changes in the known neuropeptides would reveal the structure of GHRH. Accordingly, modification of the opioid pentapeptide, metenkephalin resulted in synthesis of the first GHRP, Tyr-D-Trp-Gly-Phe-Met-NH2. However, its ability to release hGH from pituitary cells in vitro was very weak, causing many to question whether the modest results were simply contamination artifacts. Nonetheless, continued research resulted in synthesis of a group of molecules with ever increasing hGH releasing potency that ultimately was found to represent a second, yet unknown hGH secretagogue totally different from GHRH. The evolution of these molecules is presented in Figure 3.
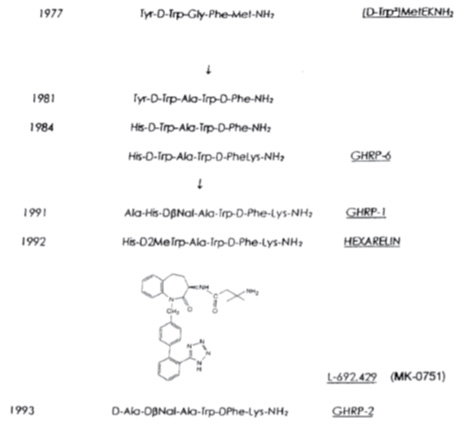
Fig 3. Evolution of molecules representing a second type of GH secretagoue (from Mueller et al. 1999)
Because of their chemical structures, these molecules were called GH releasing peptides (GHRP’s) were found to not be GHRH nor analogs of it because they bound different somatotroph receptors and employed a different intracellular second messenger (PKC not cAMP) (Fig 4).
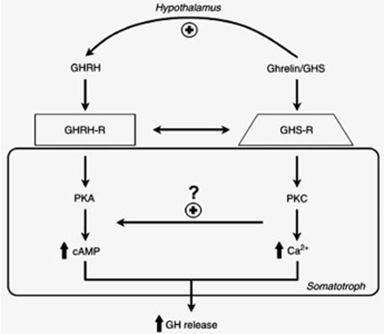
Fig 4: Separate but interactive receptors for GHRH/sermorelin and GHRP/ghrelin exist on somatotrophs using complementary intracellular messengers to “fine tune” release of GH from the pituitary gland (Lengyel, 2006)
Based upon these differences and reminiscent of the relationship between morphine and endorphins, the search for an endogenous GHRP ligand was undertaken. Finally, in 1999, more than 20 years after GHRPs were synthesized, a growth-hormone-releasing, acylated peptide from the stomach called ghrelin was discovered (Kojima et al., 1999). Although present in the stomach, ghrelin was subsequently shown to be distributed throughout the body and to display various functions in addition to GH secretion (see van der Lely et al, 2004).
Data from early studies investigating the mechanism of action and relative potency of GHRPs on GH secretion in vitro were equivocal. Not knowing at the time that there exists a synergistic relationship between GHRP and GHRH the early tests gave a significant underestimate of GHRP activity in vivo. In fact, the new molecules, especially GHRP-2 were actually capable of increasing serum hGH more than GHRH when tested in animals and especially in humans (Muller, 1999). The enhanced potency of GHRP in vivo suggested that it might be synergistic with GHRH and/or inhibitory upon somatostatin. Evidence for this fact derived from animal studies in which endogenous GHRH was absent (Pandya et al. 1998, Popovic et al., 2003, Tannenbaum et al., 2003). One definitive demonstration of GHRH/GHRP synergy was provided using mice in which the GHRH gene was destroyed (Alba et al, 2005). Chronic administration of GHRP-2 to these GHRH knockout (KO) mice, which was very potent in intact animals failed to stimulate somatotroph cell proliferation and GH secretion or to promote longitudinal growth in the KO’s. The data unequivocally demonstrated functional synergy between the two secretagouges in vivo. Since GHRP-2 failed to reverse the severe GHD caused by lack of GHRH in KO mice, it seemed that it must require at least some endogenous GHRH to be effective.
Having this information about GHRH/GHRP synergy from in vivo studies, we repeated the earlier in vitro studies, for the purpose of characterizing and differentiating the responses of primary cell cultures from rat pituitary glands to both GH secretagogues. Pituitary glands were collected from three month old Sprague Dawley rats, minced and enzymatically digested. The dispersed cells were plated, incubated for 24 hours after which time the primary cell cultures were washed with buffer and exposed to 10-10M sermorelin (GHRH), GHRP-6 or GHRH + GHRP-6 for hourly increments up to a maximum of 10 hours. GHRP-6 was readily available at the time and was used in lieu of GHRP-2 since their mechanisms are exactly the same. The GHRP’s differ only in potency. Controls were handled exactly the same as treated groups except that GH secretagogues were not added to the cell cultures. In these studies, intracellular and extracellular GH concentrations were estimated by Western blot analysis while GH messenger RNA (mRNA) was measured by Northern blot analysis.
Consistent with earlier reports, GHRP-6 alone was a very weak releaser of GH from pituitary cells compared to GHRH alone or GHRH + GHRP-6. Only a slight, statistically insignificant increase in extracellular accumulation of GH occurred in media containing cells exposed to GHRP-6 compared to unstimulated control cells (Fig 5).
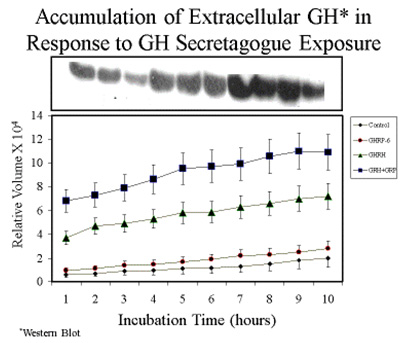
Fig 5A. Comparison of hourly increases in GH concentrations in media containing pituitary cells and GH secretagogues.
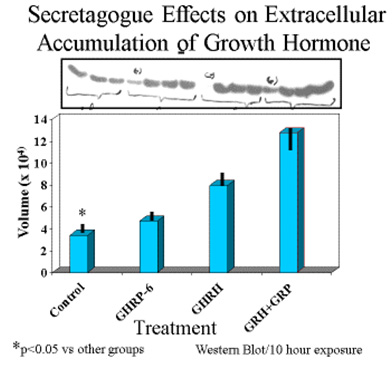
Fig 5 B: Mean concentrations of GH in media containing pituitary cells after 10 hours exposure to GH secretagogues.
On the other hand, intracellular concentrations of GH were greatest in rat pituicytes that were exposed to GHRP-6 alone, compared to their controls or to those exposed to sermorelin or GHRH + GHRP-6 (Fig 6).
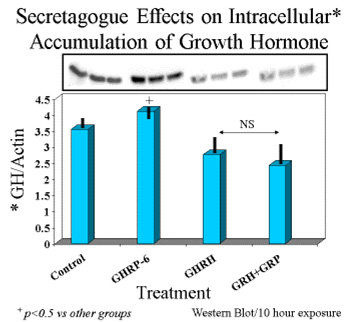
Fig. 6: Intracellular concentrations of GH in cells exposed for 10 hours to GH secretagogues alone or in combination.
These observations suggested that either translation of the GH gene was occurring more in unstimulated and GHRP-6 exposed cells than in those exposed to GHRH or GHRH + GHRP, or that the latter two groups were secreting more GH than the former two. The latter possibility was confirmed upon subsequent analysis. In fact, the ratio of extracellular to intracellular GH was the same for control and GHRP-6 exposed cells, whereas it was significantly greater in those exposed to GHRH or GHRH + GHRP-6 (Fig. 7). This observation means that there occurred an increased release of GH from cells exposed to GHRH and GHRH + GHRP-6 compared with GHRP-6 alone.
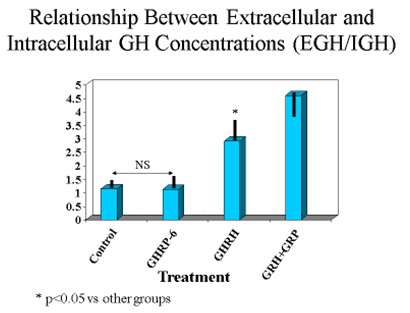
Fig 7. Ratio of extracellular to intracellular GH in pituitary cells exposed to GH secretagogues for 10 consecutive hours. Data show that the amount of GH leaving somatotrophs exposed to media or media containing GHRP alone is relatively small, whereas it is significantly greater in cells exposed to GHRH and especially in those exposed to combinations of GHRH and GHRP
Finally, exposure of rat pituicytes to GHRP-6 was associated with increased accumulation of GH mRNA approximately equal to that associated with exposure to GHRH. Mean concentrations of GH mRNA in cells exposed to GHRH + GHRP were greater than for either peptide alone (Fig 8)
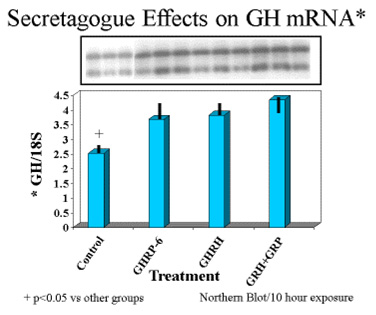
Fig.8: Effects of GHRH and GHRP alone or in combination on transcription of GH gene in pituitary cell cultures. Both secretagogues stimulated accumulation of messenger RNA compared to control conditions. Combination of both secretagoges increased mRNA production more than either secretagogue alone.
The results showed that GHRP stimulates transcription and/or translation of GH mRNA thereby increasing the gene product and GH intracellular stores. However, GHRP alone does not stimulate GH release. Instead, when GHRP is tested in vitro, GH leaves the cell passively due to increasing intracellular GH concentrations that eventually exceed the threshold for spontaneous release. This conclusion is supported by the fact that the ratio of extracellular to intracellular GH was the same in control and GHRP-6 exposed cells. Thus, unlike GHRH, which actively promotes GH release, GHRPs have minimal effect on GH secretion, but significantly affect its production. In this sense, GHRP-6 is not a secretagogue, per se. This fact explains why the GHRPs appear inactive when tested alone in vitro or in animals lacking endogenous GHRH. However, GHRP enhances the secretagogue effect of GHRH/sermorelin by increasing intracellular stores of GH which provides a larger pool for active release by sermorelin. GHRPs also directly enhance the GHRH secretory function, as indicated by the robust release of GH in response to initial exposure. In any event, these results in conjunction with in vivo studies demonstrate that for GHRPs to be clinically effective, the sermorelin/GHRH receptor must be active. Conversely, sermorelin/GHRH action on somatotroph cells is not influenced by blockage of the GHRP receptor demonstrating the primary and secondary/modulatory roles of GHRH and GHRP’s, respectively (Kamegai et al., 2004). This information is particularly relevant to clinical applications for GHRP-2 in aging because GHRH titers decline but do not disappear completely as we grow old. Thus, GHRP-2 can be used by itself in early, age-associated hGH insufficiency to restore effectiveness of the remaining endogenous GHRH through synergy and thereby to promote recrudescence of the hGH neuroendocrine axis and oppose maladaptive effects of senescence. However later in life, when levels of endogenous GHRH become critically low, then an appropriate clinical approach to restoring GH neuroendocrine activity would be to provide combinations of sermorelin and GHRP-2 as seen in (Figure 9).
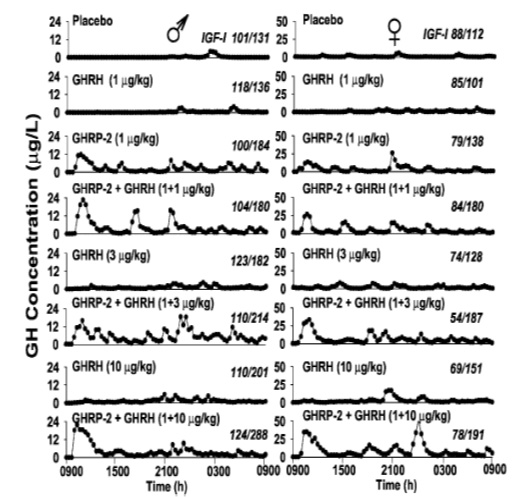
Fig 9: Effects of 24 hour infusion of GH Secretagogues on GH secretory patterns and IGF-1 levels in elderly men and women. GHRP-2 has a greater effect than GHRH on increasing the amplitude of GH pulses. Attenuation of these pulses is a significant detrimental change associated with aging. (Bowers, et al, 2004)
In addition to its synergistic effect with GHRH, GHRP-2 simulates the normal, physiological secretory profiles of hGH which is markedly pulsatile in all species that have been studied (see Muller et al., 1999). The pulsatile pattern of secretion which is essential for hGH efficacy becomes blunted with age and it eventually ceases. This effect is more due to the dynamics of endogenous GHRH secretion than to its absence from the body. Relevant to the previously mentioned synergistic effect of GHRP-2 and endogenous GHRH is the fact that GHRP-2 amplifies the naturally occurring pulsatile GH secretion that becomes diminished as the relative concentrations of GHRH decline with age (Figure 8). In the short term, i.e., over a period of 24 hours, Bowers et al (2004) showed that the combination of GHRH and GHRP gives best return to pulsatility, but over the longer term as pituitary recrudescence occurs, then the need to administer exogenous GHRH along with GHRP-2 may become less significant. This is true except in cases of severe GHRH deficiency. Then oral GHRP-2 (Walker, 1990) can be supplemented with sermorelin sublingual for chronic therapy.
GHRP-2 Safety: Published reports of the beneficial effects of potent ghrelin analogs such as GHRP-2 include its ability to stimulate appetite and growth (Mericq, V.N., et al. 1998; Laferrere, B. et al., 2004), control gastric motility and secretion, modulate pancreatic and immune functions, glucose metabolism, cardiovascular performance, sleep and behavior (see Van Der Lely, A.J. et al. 2004). To be an effective agent for opposing the unending progression of senescence, GHRP-2 should be used as a daily, on-going component of therapy. However, this recommendation for chronic administration of the peptide raises the question of compliance and especially of safety.
Extensive literature review failed to identify any reports of general, reproductive or cancer-causing toxicity for GHRP-2 in animals or humans. For the most part, lack of toxicity has been reported in peer-reviewed studies performed for therapeutic purposes (Bowers et al. 2004; Pihoker et al., 1998; Van den Berghe et al., 1997) even when administered at relatively high daily doses for as long a year. A study in pre-pubertal children using 900 µg/kg GHRP-2 orally, twice daily was without any adverse effects (Mericq et al., 2003) and it has been chronically administered intranasally without any evidence of side effects (Pihoker et al., 1997). Thus, while effective for its intended clinical benefits, the risk of dose-related adverse events occurring at the therapeutic doses was non-existent. In conclusion, the findings of studies reported herein, taken in conjunction with a history of three decades use in human subjects without a single report of toxicity in the peer-reviewed literature, support the view that the peptide is safe and effective for age management.
GHRP-2 Efficacy: While GHRP-2 mimics many of the physiological effects of ghrelin, it has received greatest attention clinically for its potent hGH releasing activity. One possible application for that effect is treatment of sarcopenia that occurs in everyone as they grow old. Sarcopenia is a serious and global threat to health and quality of life in aging populations. Characteristic of sarcopenia is spontaneous and progressive muscle weakness with actual loss of lean body mass that contributes significantly to frailty, loss of functional mobility and independence as well as increased risk for development of intrinsic disease (Doherty T., 2003; Roubenoff R., 2001; Roubenoff R and Hughes V.A., 2000). In fact, erosion of skeletal muscle mass occurs in as much as 30% of the population beyond the age of 60 years. While sarcopenia is known to result from multiple factors, including age-related GH insufficiency, the need to develop effective and safe endocrine therapies to oppose its progression is obvious.
The possible benefits of GHRP-2 were tested in a double blinded, placebo controlled clinical trial involving 75 healthy subjects ranging in age from 40 to 68 years. The following data resulted from that 90 day study (Walker and Saini, 2014).
Mean concentrations of serum insulin-like growth factor-1 (IGF-1) significantly increased from 103.54 ± 1.94 ng/mL at baseline to 120.47 ± 2.10 ng/mL after 90 days of treatment. In contrast, there were no significant changes in subjects receiving placebo.
While body Mass Index (BMI) and total body water did not change significantly in either treatment or placebo groups, total body fat was significantly decreased from 34.46 ± 1.23 % at baseline to 31.31 ± 1.11 % at the end of GHRP treatment. Visceral fat decreased from 11.84 ± 0.66 % at baseline to10.15 ± 0.67 % after 90 days of GHRP-2 administration. There were no changes in total or visceral body fat within the placebo group.
Muscle mass was significantly increased from 41.88 ± 1.28 Kg at baseline to 44.13 ± 1.24 Kg , while forced vital capacity (FVC) significantly increased from 71.84 ± 3.18 % at baseline to 83.77 ± 3.70 % at day 90. There were no changes in FVC in the placebo group throughout the course of study.
Finally, using Quality of Life (QoL) measures, GHRP-2 treated patients reported having increased energy, improved work ability/capacity, increased confidence, improved physical fitness, increased resistance towards illness, increased sleep duration and overall feelings of happiness. A few of subjects reported having reduced back pain and ankle pain while others remarked about improvement in skin texture and radiance.
Conclusions: Because of their effects on body composition, GH secretion, appetite and energy metabolism the GH secretagogues such as GHRP-2 and sermorelin are appropriate for various applications in age management including treatment of sarcopenia, a major threat to health and vitality in the elderly (Doherty, T.J., 2003). Thus, the need to develop effective pharmacological interventions that may prevent or partially reverse sarcopenia, is evident. Since GH secretagogues are known to stimulate production of GH by the pituitary and also to restore natural, physiological, episodic release of the hormone, they have the potential to complement resistance exercise as well as amino acid and protein supplements as another beneficial factor for opposing many age-associated dysfunctions.
Literature Cited
Alba M, Fintini D, Bowers CY, Parlow AF, Salvatori R. Effects of long-term treatment with hormone-releasing peptide-2 in the GHRH knockout mouse. Am J Physiol Endocrinol Metab 2005, 289:E762-E767. doi:10.1152/ajpendo.00203.2005
Bowers CY, Granda R, Mohan S, Kuipers J, Baylink D, Veldhuis J. Sustained elevation of pulsatile growth hormone secretion and IGF-1 concentratinos during subcutaneous administration of GHRP-2 in older men and women. J Clin Endo Metab 2004, 89:2290-2300.
Doherty, T.J., 2003. Invited Review: Aging and Sarcopenia. J. Appl. Physiol. 95, 1717–1727; doi:10.1152/japplphysiol.00347
Frohman LA, Szabo M, Berelowitz M, Stachura ME. Partial purification and characterization of a peptide with growth hormone-releasing activity from extrapituitary tumors in patients with acromegaly. J Clin Invest. 1980, 65(1):43–54.
Kamegai J, Tamura H, Shimuzu T, Ishii S, Tatsuguchi A, Sugihara H,
Oikawa S, and Kineman RD. The role of pituitary ghrelin in growth
hormone (GH) secretion: GH releasing hormone-dependent regulation of
pituitary ghrelin gene expression and peptide content. Endocrinology 2004,145:
3731–3738.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach Nature 1999, 402: 656-660 doi:10.1038/45230
LaFerrere, B., Abraham, C., Russell, C.D., Bowers, C.Y., 2004. Growth hormone releasing peptide-2 (GHRP-2) like ghrelin, increases food intake in healthy men. J. Clin. Endo. Metab. 90(2), 611-614; doi 10.1210/jc.2004-1719.
Lengyel MJ. Novel mechanisms of growth hormone regulation: growth hormone releasing peptides and ghrelin. Braz J Med Bio Res 2006,39:1003-1011
Ling N, Esch P, Bohlen P, Brazeau P, Wehrenberg W, Guillemin R. Isolation, primary structure and synthesis of human hypothalamic somatocrinin: growth hormone releasing factor. Proc Natl Acad. Sci USA 1984, 81: 4302-4306.
Mericq, V., Cassorla, F., Bowers, C.Y., Avila, A., Gonen, B., Merriam, G.R., 2003. Changes in appetite and body weight in response to long-term oral administration of the ghrelin agonist GHRP-2 in growth hormone deficient children. J. Pediatr. Endocrinol. Metab. 7, 981-5.
Mericq, V.N., Cassorla, F., Salazar, T., Avila, A., Iniguez, G., Bowers, C.Y., Merriam, G.R 1998. Effects of Eight Months Treatment with Graded Doses of a Growth Hormone (GH)-Releasing Peptide in GH-Deficient Children. J. Clin. Endocrinol. Metab. 83, 2355–2360.
Muller E, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999, 79: 512-575.
Pandya N, DeMott-Friberg R, Bowers CY, Barkan AL, Jaffe CA.
Growth hormone (GH)-releasing peptide-6 requires endogenous hypothalamic
GH-releasing hormone for maximal GH stimulation. J Clin Endocrinol
Metab 1998, 83: 1186–1189.
Pihoker, C., Badger, T.M., Reynolds, G.A., Bowers, C.Y. 1997. Treatment effects of intranasal growth hormone releasing peptide-2 in children with short stature. J. Endocrinol. 155, 79-86.
Pihoker, C., Kearns, G.L., French, D., Bowers, C.Y., 1998. Pharmacokinetics and Pharmacodynamics of Growth Hormone-Releasing Peptide-2: A Phase I Study in Children. J. Clin. Endocrinol. Metab. 83, 1168–1172.
Popovic V, Miljic D, Damjanovic S, Arvat F, Ghigo E, Dieguez C, and
Casanueva FF. Ghrelin main action on the regulation of growth hormone
release is exerted at hypothalamic level. J Clin Endocrinol Metab 2003, 88:
3450–3453.
Roubenoff, R., 2001. Origins and clinical relevance of sarcopenia.
Can. J. Appl. Physiol. 26, 78–89.
Roubenoff, R., Hughes, V.A., 2000. Sarcopenia: current concepts.
- Gerontol. A. Biol. Sci. Med. Sci. 55, M716–M724.
Rudman D, Feller AG, Nagraj HS, et al. 1990. Effects of human growth
hormone in men over 60 years old. N Engl J Med 1990, 323:1-6.
Tannenbaum GS, Epelbaum J, and Bowers CY. Interrelationship between
the novel peptide ghrelin and somatostatin/growth hormone releasing
hormone in regulation of pulsatile growth hormone secretion. Endocrinology
2003, 144: 967–974.
Van den Berghe, G., de Zegher, F., Veldhuis, J.D., Wouters, P., Awouters, M., Verbruggen, W., Schetz, M., Verwaest, C., Lauwers, P., Bouillon, R., Bowers, C.Y.,
- The Somatotropic Axis in Critical Illness: Effect of Continuous Growth Hormone (GH)-Releasing Hormone and GH-Releasing Peptide-2 Infusion J. Clin. Endocrinol. Metab. 82, 590–599.
van der Lely A, Tschop M, Heiman M, Ghigo E. Biological, physiological, pathophysiological and pharmacological aspects of ghrelin. Endo Rev. 2004, 25:426-457.
Walker RF: Sermorelin: A better approach to management of adult-onset growth
hormone insufficiency? Clin Interventions Aging, 2006: 1(4):1-2.
Walker RF, Codd EE, Barone FC, Nelson AH, Goodwin T, Campbell SA. Oral activity of the growth hormone releasing peptide His-D-Trp-Ala-Trp-D-Phe-Lys-NH2 in rats, dogs and monkeys. Life Sciences 1990,47:29-36.
Walker RF, Saini R. A 90 day Clinical Trial of a Medical Food for
Treatment and Prevention of Age-Related Sarcopenia. Drug Design Devel Ther 2014 (submitted for peer review).





