
The role of glycation in aging
November 28th, 2016The role of glycation in aging
By Marios Kyriazis, M.D.
Even people who have no training in chemistry or biology, but who are interested in the aging process, have heard of the Maillard reaction. This is a chemical reaction between proteins (specifically, amino acids) and sugar molecules, a process called glycation.
The reaction was described for the first time in 1912, anology, but who are interested in the aging process, have heard of the Maillard reaction. This is a chemical reaction between proteins (specifically, amino acids) and sugar moleculd it takes its name from the French chemist Louis-Camille Maillard. Over the years, this reaction has been studied in detail. Among other things, it plays a crucial role during the process of aging.
The end-products of this reaction are called AGEs (Advanced Glycation End-products). AGEs react with other molecules in the body to bring about damage to our tissues. AGEs can be of either high or low molecular weight, and interact with receptors on the surface of cells (appropriately called RAGEs— i.e. Receptors for AGEs) causing a variety of damage.
Examples of AGE-associated involvement in age-related complications include diabetes, stiffening of the arteries (with increased risk of high blood pressure and possible stroke), cataracts, and skin damage. AGEs can originate externally (from cooked food, for example), or internally, through free radical reactions, ionizing radiation, and other age-related processes.
Only low molecular weight AGEs are absorbed from the gut. Glycation reactions may also happen between sugar molecules and DNA; not just between sugar and amino acids. This shows that the potential for glycation damage is considerable. Therefore, research into finding ways to lessen the impact of glycation has a high priority.
Several agents capable of reversing or retarding the process of glycation include alagebrium (ALT 711) and aminoguanidine. I discussed many other anti-glycators in a previous Aging Matters™ edition (see box 1).
****************************************************************
BOX 1
Some anti-glycators (other than alagebrium and aminoguanidine) are:
- Carnosine
- Thiazolidinedione’s such as Pioglitazone (Actos), Rosiglitazone (Avandia), and Lobeglitazone (Duvie) – there are certain restrictions or warnings relating to these
- Pyridoxamine
- Benfotiamine (a fat-soluble form of thiamine [Vitamin B1])
- Angiotensin converting enzyme inhibitors (ACE inhibitors) (Enalapril, Captopril, Lisinopril and Perindopril are the commonest in clinical use) and angiotensin receptor blockers (ARBs).
- Metformin
- Not yet available for clinical use:
- ALT-946
- OPB-9195
- Tenilsetam
- Others less well-studied such as LR-90, TM2002, sRAGE and PEDF (1)
- Natural anti-glycating compounds include polyphenols, anthocyanins and other flavonoids.
****************************************************************************
Alagebrium (ALT 711)
ALT 711–also known as thiazolium chloride—is one of the best-known crosslink breakers in existence, although its development has been hampered by financial and other issues. Alagebrium diminishes the effect of AGEs and has positive effects in diabetes, arterial stiffening, and age-related myocardial disease (2).
One significant effect of alagebrium is that it lessens the impact of atherosclerosis in diabetes. Diabetes is associated with increased production of AGEs and risk of atherosclerosis. Alagebrium or pyridoxamine (another anti-glycator) inhibited the accumulation of AGEs and slowed down the progression of atherosclerosis in mice. (3) Other benefits of Alagebrium include improved memory function and delayed neuronal degeneration in diabetic animals, suggesting that alagebrium has a potential neuro-protective effect (4).
Methylglyoxal is a by-product of the glycation reaction. Methylglyoxal induces AGE formation, and results in increased inflammation and cell death through apoptosis. Many studies have concentrated on ways to reduce methylglyoxal, with variable success, such as by supplementation with carnosine. Alagebrium, in particular, when applied to rat heart muscles, reduced methylglyoxal and subsequently also reduced excessive inflammation and cell death (5).
Alagebrium may be acting as both a preventative as well as a curative agent.
*********************************************************
BOX 2
There are several chemical entities forming part of the group of Advanced Glycation End-products (AGEs). Some examples are:
- N-ε-carboxy-ethyl-lysine (CEL) and N-ε-carboxy-methyl-lysine (CML)
- Imidazolone
- Methyl-glyoxal-lysine dimer (MOLD) and glyoxal-lysine dimer (GOLD)
- Pyrraline
- Pentosidine
- Glucosepane
- Amadori products, (although these are more precisely described as intermediate products, leading to the formation of other AGEs)
*******************************************************************************
From the above list (as per box 2), we know that Alagebrium is effective against pentosidine (6), carboxy-methyl-lysine (CML) (7) and methylglyoxal, (5) but not against glucosepane. This is unfortunate, because glucosepane is the most abundant glycation product in humans.
The issue with glucosepane
Glucosepane is a complex protein resulting from the glycation process (see figure 1). Although present in all organisms, it plays a significant role in human aging (8). Together with methylglyoxal, glucosepane is a marker of glycation, and its presence is used to monitor glycation damage (9). In fact, methylglyoxal is a precursor of glucosepane. It has been suggested that glucosepane makes up the vast majority of cross-links in older people and there is no efficient way to eliminate this molecule from our body (although several researchers have their doubts about the validity of this statement). Currently, there is a race to discover an efficient compound that can eliminate glucosepane from our aging bodies, in the hope that this will result in a reduction of age-related pathology.
Figure 1: The glucosepane molecule
Aminoguanidine
Also known as Pimagedine, this compound is an inhibitor of Nitric Oxide (NO) synthase and an antiglycator. It reduces AGE concentrations and has the potential to reduce age-related degeneration. Aminoguanidine as well as pyridoxamine are potent carbonyl and free radical scavengers.
During the process of glycation, intermediaries such as dicarbonyl groups are formed. However, aminoguanidine has the ability to inactivate these dicarbonyl groups (10) and helps prevent complications of diabetes such as kidney damage, vascular disease and neuropathy.
The age-related glycation of collagen causes a series of skin changes (wrinkles and skin thickening, for example). Aminoguanidine prevents these changes in the laboratory environment, and clinical research in human age-related skin changes is underway. Recent research (11) shows that aminoguanidine applied as a skin cream can reduce the levels of Nε-carboxymethyl lysine (CML) (see box 2) and may be useful in delaying skin aging.
Aminoguanidine also increases the phagocyte response and improves antibacterial actions in white blood cells, reducing inflammation (12). This mechanism is believed to be via aminoguanidine’s anti-glycating action which reduces oxidation and normalizes the function of signaling pathways. It is therefore becoming clear that aminoguanidine has a range of anti-glycating activities, working at multiple levels, (as shown in figure 2).
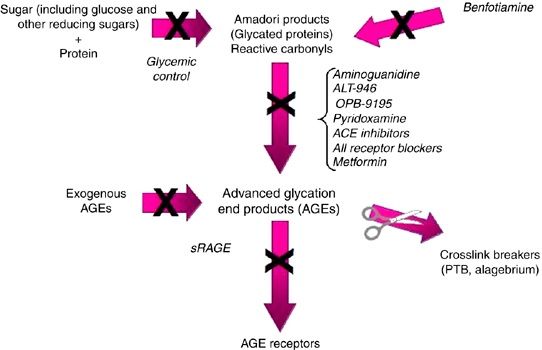
Figure 2: A simplified image showing the different pathways and compounds involved in anti-glycation.
Conclusion
Although certain researchers are not entirely convinced of the clinical effectiveness of anti-glycators (13) others are satisfied that there is significant potential. Overall, the information available appears optimistic, with many thousands of people using the various anti-glycation compounds.
References
- Nenna A. et al. Pharmacologic Approaches Against Advanced Glycation End Products (AGEs) in Diabetic Cardiovascular Disease. Res Cardiovasc Med. 2015 May 23;4(2):e26949
- Krautwald M. et al. The advanced glycation end product-lowering agent ALT-711 is a low-affinity inhibitor of thiamine diphosphokinase. Rejuvenation Res. 2011 Aug;14(4):383-91
- Watson AM et al. Delayed intervention with AGE inhibitors attenuates the progression of diabetes-accelerated atherosclerosis in diabetic apolipoprotein E knockout mice. Diabetologia. 2011 Mar;54(3):681-9
- Zakaria MN, El-Bassossy HM, Barakat W. Targeting AGEs Signaling Ameliorates Central Nervous System Diabetic Complications in Rats. Adv Pharmacol Sci. 2015;2015:346259
- Dhar A, Dhar I, Bhat A, Desai KM. Alagebrium attenuates methylglyoxal induced oxidative stress and AGE formation in H9C2 cardiac myocytes. Life Sci. 2016 Feb 1;146:8-14
- Park J. et al. Renoprotective antioxidant effect of alagebrium in experimental diabetes. Nephrol Dial Transplant. 2011 Nov;26(11):3474-84
- Peppa M, et al. J Prevention and reversal of diabetic nephropathy in db/db mice treated with alagebrium (ALT-711). Am J Nephrol. 2006;26(5):430-6
- Draghici C, Wang T, Spiegel DA. Concise total synthesis of glucosepane. Science. 2015 Oct 16;350(6258):294-8. doi: 10.1126/science.aac9655
- Monnier VM, Genuth S, Sell DR. The pecking order of skin Advanced Glycation Endproducts (AGEs) as long-term markers of glycemic damage and risk factors for micro- and subclinical macrovascular disease progression in Type 1 diabetes. Glycoconj J. 2016 Jun 25. [Epub ahead of print]
- Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31–40
- Lee KH. et al. Molecular characterization of glycation-associated skin ageing: An alternative skin model to study topical anti-glycation activity in vitro of a cosmeceutical and pharmaceutical formulation. Br J Dermatol. 2016 Jul 1. doi: 10.1111/bjd.14832. [Epub ahead of print]
- de Souza Ferreira C. et al. Aminoguanidine treatment increased NOX2 response in diabetic rats: Improved phagocytosis and killing of Candida albicans by neutrophils. Eur J Pharmacol. 2016 Feb 5;772:83-91
- Engelen L, Stehouwer CD, Schalkwijk CG. Current therapeutic interventions in the glycation pathway: evidence from clinical studies. Diabetes Obes Metab. 2013 Aug;15(8):677-89







