The battle for effective antibiotics and antivirals continues The 1st Line™ story (so far)
February 20th, 2020By Richard Stead
Background
1st Line is a supplement that creates OSCN molecules in a glass of water, (otherwise known as oxythiocynate ions). These molecules are found naturally in tears, saliva and mother’s milk. They are literally the ‘first line of immune defence’ and have been proven against all manner of viruses and pathogens.
Professor Paul Clayton wrote about them (with references) in the Aging Matters™ magazine (Issue 1, 2013).
The problem with OSCN are that they have a very short half-life, a matter of less than a hour, which means they have to be generated almost on demand. It is estimated that a healthy body could produce about 25 mg of OSCN every day. As you can imagine that makes a supplement containing OSCN very difficult to create, especially in terms of delivering them to the market and a user.
Richard Stead, (the author of this article) was the clever chemist who worked out a way by means of a kit, (known as 1st Line™) that makes OSCN in a glass of water, it can then be consumed/ drunk immediately afterward. The method is very simple and providing that each of the four agents are mixed in the right order, (they are clearly marked as 1, 2, 3 and 4), then 25 mg of OSCN can be consumed/ drunk as a supplement. Note that there is that there is no discernible smell nor taste.
The fact is that antibiotics are well-known to be failing and there are very poor choices for anti-virals too, so one would have thought that OSCN would have garnered a lot of attention.
In this article Richard Stead describes the difficult processes and choices he has been through, ever since IAS introduced the concept of OSCN in 2009.
It is amazing how fast time goes, indeed IAS interviewed me 10-years ago (that video is on You Tube). Since then, a lot has happened- and lot that should have happened has NOT happened!
Sales of 1st Line™ have been steady but the hoped-for explosion has not been achieved. I thank IAS and the Aging Matters™ magazine for their efforts to support the presentation of 1st Line™ and for being an early adopter of this technology.
The first years of euphoria were after the OSCN laboratory results and they excited the practitioners. Alas, we have not been able to publish many more results to give those practitioners wider reasons to look at 1st Line™ to help their patients. My apologies to you for this paucity of support, but please allow me to explain the story to date and where we are now.

Disappointments and successes
OSCN has been shown to be effective against pathogens (bacterial, viral and fungal). Nature designed the ion to be such a broad-spectrum antimicrobial. With the enormous number of academic papers verifying this scope of efficacy, I applied for UK Government and EU funding to proceed to clinical trial, but every application was unsuccessful.
The main reason for rejection was described as “not novel” since OSCN has been in the literature for about 40 years. However, in that time no proposal to use it in humans or animals was ever put forward! Thus, I was of the opinion that novelty had been proposed and that the kit design is unique- in that you create the effective substance at the point of use- again novelty.
Antibiotic resistance
The biggest surprise to me was that our proposed application, which offered a viable alternative to some antibiotics, (at a time when the world is worried about pathogens becoming ABR (antibiotic resistant) did not receive any feedback at all.
Maybe my applications were not good enough? But why did nobody think that I might just have one of the answers to the growing tsunami of ABR? Some of the more virulent pathogens are predicted to cause the deaths of 500,000 people over the next 10 years! There are currently in excess of 20,000 per year dying this way in the EU and a similar number in the USA. I thought I might get a call from somebody who wanted to review my technology and then maybe tell me if it was of any use.
Instead I faced silence.
Investment requirements
Putting these disappointments to one side, we then presented a request for funding to a range of brokers to find corporate or private funding. The request was for a modest US$7 million for some lab work and a trial involving about 60 Influenza sufferers. I’m sad to say that there was not one taker.
The reason was simple. Their investment returns would be too low compared to Chronic Conditions. Investors who want products for cardiac, cancer and diabetic conditions etc. Ergo, they prefer protocols that are taken by the patient every day for the rest of their lives, not curing them of course, but managing the disease- or more accurately, controlling their symptoms to an acceptable level of ill-health.
Thus, it is hard to dispute the financial logic behind my failure to gain investors.
So, it was up to me to go it alone and see what I could achieve within my own financial capabilities. A strategy was created, and actions planned. I decided we needed to achieve 4 things:
- A trial/ study for a condition which have enough potential to generate millions of unit of sales. (A number that should be achievable in a healthcare environment).
- A place to perform the study/ trial.
- Create the OSCN using food grade materials, thereby being able to market it as a food supplement rather than a pharmaceutical product. Since all the components are food approved or have GRAS status, we have achieved this criterion.
- Price the product at a reasonable value so that it would be respected by users.
The outcome of these criteria is that we settled on making Upper Respiratory Tract (URI) conditions our focus. Below I will explain how this decision was arrived at.
Human Study
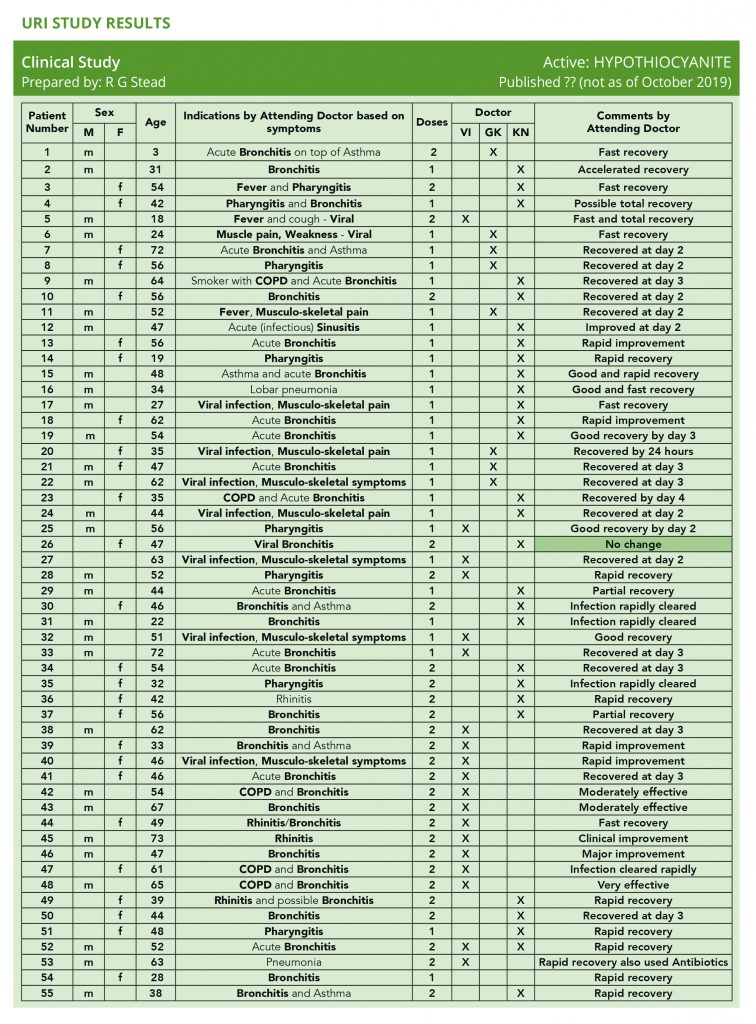
A hospital in Hungary conducted this study and the results (see addendum 1) were wonderful – with an unexpected bonus outcome, see addendum 2.
The biggest obstacle to this strategy was satisfying the Hospital’s Ethics Committee. I am indebted to the Head of the Hospital Dr. Thomas, and to Dr. Paul Clayton and Dr. Szabolcs Ladi for their belief in OSCN and their support in presenting details to the Committee, and to their desire to find answers to ABR together with their intelligence in helping identify URIs as the target condition. In our submission we stated that should the patient see/feel no benefit then we would offer them antibiotics. We were treating patients that did not have life-threatening conditions, so the Ethics Committee were able to give the go-ahead.
Why URIs?
Some statistics in Healthcare within the developed world surprised me:
- URIs have more antibiotics prescribed than for any other indication, (this is ridiculous and shameful when it is known that 80% of URIs are of viral causation).
- Each year people suffer on average two URIs of varying severity.
- Typically, a URI will last 2-5 days.
- URIs are the biggest cause of time off work.
- Lost time off work is an average of more than 2 days per incident. That time lost in the USA amounts to over 100 million days per year.
- The cost to the US economy is estimated at several Billion Dollars.
- The employees can lose earnings.
- There is the consideration of a drop-in productivity for the company.
- There is the implicate cost to insurance and the insurers.
- There is a loss of tax/ income for the Government.
- Current healthcare costs in this area are enormous – hospital time, medications, etc
So, if we can do something to reduce the number of working days lost, we offer savings to all interested parties. Even if it is only a reduction of one day, then an effective antiviral is a unique achievement.
Summary
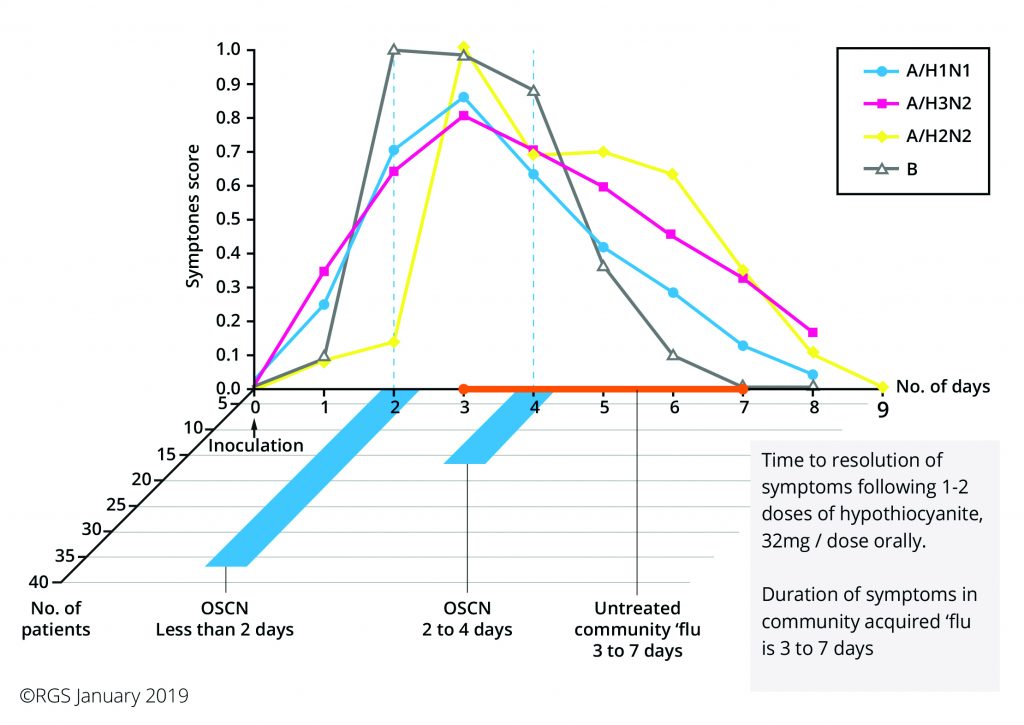
We are almost there, as you can see from the attached summary of the study results; the results are quite remarkable with an 80% success in symptom reduction within 24-48 hours and a faster than usual rate of recovery. This was achieved with one dose of 1st Line™ which was sometimes repeated the following day.
I am convinced that what we have achieved is to offer our bodies a natural boost to our immune defence. In so doing it is a faster alternative to overcome the pathogens.
Education is, as always, an important part of my work as is the support for Practitioners. One point that became clear after talking with the three Doctors involved in the study, was that at whatever stage the OSCN was delivered there was a positive and sustained improvement in symptoms.
The message is clear, take 1st Line™ as soon as any symptoms appear.
Other OSCN uses
There are other indications/conditions with which OSCN is proving to be successful, especially the Gut.
A dysbiotic Gut can cause all sorts of immune problems and especially its links with the Brain, affecting cognitive and mental issues. Almost all people that suffer with mental conditions also have bad guts. Is the brain affecting the Gut, or is it the Gut affecting the brain? OSCN has a positive effect upon a dysbiotic gut. Work on this use of OSCN currently takes up most of my time. It is my view that destroying pathogens in the gut is important, although 1st Line™ is an initial step in taking the patient towards a healthy situation linked with a sensitive and validated probiotic and prebiotic protocol.
URI Study Results (addendum 1)
The Sepsis bonus
There was an exciting follow-up story after the successful study. Throughout the Hungarian hospital, apparently, the scale of the success of our study was becoming well-known. A few weeks after the study had been completed the head of ICU approached Dr. Ladi to ask if he could have some of the OSCN kits.
He had a female patient with Sepsis and, after several weeks during which all antibiotics had failed, she was in a coma with her body starting to shut down. X-rays showed her lungs were blank, covered with infection, she relied upon a machine to oxygenate her blood. Her prognosis was bad. We managed to get product administered to her within 3 days. The Ethics committee had no difficulty in allowing OSCN to be administered since the success of the earlier study and a “last resort” scenario made for a quick decision.
It was rewarded with a positive response by the patient. The day after her first dose, (delivered by feeding tube) X-rays showed some clear patches in her lungs and within a few days she was free of infection, able to breathe unaided. Sadly, she never recovered consciousness. Alas, her body was unable to recover from the late stage process and she died infection free.
This success led to two more uses of OSCN- to fight Sepsis within the same hospital. A baby and a young man in his thirties. In both cases the OSCN was delivered orally and was effective at removing the infection within a matter of days, in both cases leading to a full recovery and who, fortunately for them since they were allowed OSCN before they arrived at the “last resort” stage.
Note: A paper is to be published soon and it will include all these clinical details and results, (see addendum 2 at end for details).
A request
In the years since the first article on OSCN, it has been used successfully for several conditions. All the successes have been reported to me are second-hand anecdotes. It is good to know of successes and anecdotes are better than nothing. I have not been told of indications for which OSCN was not successful, and I am sure there must have been some.
My request is that when Practitioners use or prescribe 1st Line™ they inform us of all or any of the outcomes. I will prepare and share the positive and negative outcomes with all interested parties. Of course, all such reports must be anonymised, we only need: Age, sex, weight, indication, protocol (including other medications involved) and the outcome. Thanks in advance for your consideration and support.
Should one or more Practitioners wish to carry out a small study on an indication, I am happy to consider some form of support. The results of such a study to be used for presentations and made generally available to other interested parties.
Conclusion
The dogma and the vested interests that are in place today make it very difficult to bring a natural agent such as OSCN to the general marketplace. This is even in-spite of the marvellous properties it may hold in terms of treatment prospects and paucity of side-effects and contraindications.
As an inventor, my hope is that OSCN (1st Line™) will one day become mainstream and not just remain in a specialist realm for those who ‘happen to know.’ But as is so often in life, each of us does the best we can and Que Sera Sera.
I trust that this article is published in time to help you through the next flu and URI season.
Addendum 2