Pyridoxamine: A universal weapon against aging
November 26th, 2016Pyridoxamine: A universal weapon against aging
By Karen Kaufmann, MS
We are constantly being bombarded with messages from the media, the department of public health, and the World Health Organization (WHO) about how overweight and sedentary we have become. The incidence of obesity has reached epidemic proportions. Our growing waistlines coupled with our sedentary lifestyles have put us at risk for developing Type 2 diabetes as well as a host of other chronic degenerative diseases. I raise this issue, because in the face of all this public discussion, it is easy to ignore the public health warnings, particularly if you are an individual of normal weight and consider yourself at low risk of developing diabetes.
Actually, when one looks at the disease Diabetes Mellitus (DM), it is apparent that what is truly occurring is an accelerated form of aging. Aging is occurring in all of us. It is important to make note of the many microvascular and macrovascular complications of diabetes because these complications provide us with clues from which we can form a logical and rational anti-aging protocol.
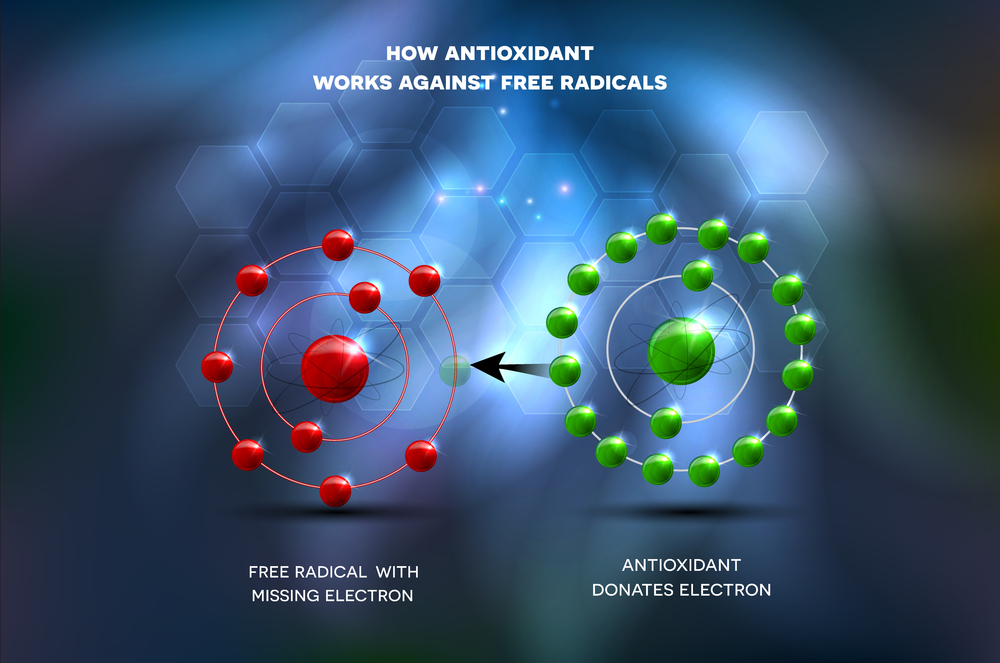
There are a number of different theories of aging. One of the oldest and best known theories is Denham Harman’s ‘free radical’ theory of aging which dates back to 1956. This theory posits that unpaired oxygen electrons, which are produced during aerobic respiration, cause cumulative oxidative damage, resulting in the effects of aging and death.[1] Harman posited that endogenous oxygen radical generation occurs within the body, as a by product of enzymatic redox chemistry. Therefore the necessary ingredient for life – oxygen – is a double edged sword. While we can’t live without breathing in oxygen, we cannot utilize oxygen without it damaging our cells.
Another theory of aging is Johan Bjorksten’s cross-linking theory of aging. The cross-linking theory of aging and the free radical theory of aging are not mutually exclusive, but synergistic.[2] [3] Ironically the cross-linking theory of aging is actually based upon processes described by the food chemist Louis Camille Maillard in 1912 who outlined the non-enzymatic chemical reactions between proteins and carbohydrates that cause foods to turn brown.
This process also occurs in the body when a reduced sugar (usually glucose) attaches to a protein. An intermediate reversible product is formed. That product is a Schiff base. As the protein/glucose complex continues to be exposed to additional sugar a more stable, less reversible complex forms. That complex is known as an Amadori product. Amadori products further degrade into a number of highly reactive carbonyl compounds. These compounds are known as Advanced Glycation End products (AGEs) or when lipids or fats are involved Advanced Lipoxidation End products (ALEs). AGE/ALEs go on to react with other fats, proteins, amino acids, nucleic acids and a variety of other cells.[4] AGEs in tissues increase the rate of free radical production, cause tissue injury, inflammation, and can deposit anywhere. For example, when AGEs attach to LDL cholesterol, the LDL cholesterol is rapidly oxidized and is more likely to deposit within a blood vessel thereby contributing to plaque formation and atherosclerosis. AGEs form at an accelerated rate in hyperglycemia, diabetes, and metabolic syndrome.
However, AGEs are a universal symptoms of aging. They can deposit in any organ or tissue, including the skin, the lungs, the blood vessels, the lens of the eye, the neurons in the brain, and in the filtering mechanism in the kidney.[5] [6] Whenever and wherever AGEs deposit, the normal functioning of that organ, tissue or cell is compromised. There is evidence that AGEs can bind with DNA and have a mutagenic effect (contributing to the risk of cancer and autoimmune disease).
When we think of the formation of AGEs and ALEs we generally think of them forming endogenously as a result of the glucose that is already within the body. We often forget that there are also dietary AGEs and ALEs. Our exposure to AGEs and ALEs occurs as an end result of normal metabolism. However we can also be contributing to the problem through the intake of dietary, exogenous AGEs.[7] So, no matter who we are, how fit we are, how thin we are, we cannot escape the effects of these cross linked proteins.
Pyridoxamine
Vitamin B6 exists in 3 naturally occurring forms: pyridoxine, pyridoxal and pyridoxamine (PM). Pyridoxine is the form most commonly seen in supplements. However, each can be phosphorylated at the 5 position. Pyridoxal 5’ phosphate (PLP) and pyridoxamine 5’ phosphate (PMP) are the active coenzyme forms. Pyridoxine is found in plant sources. Pyridoxamine and pyridoxal are found in animal sources where they exist mainly in their phosphorylated forms.[8] All 3 forms have some ability to function as anti-glycation agents inhibiting the formation of AGE/ALEs, but it is pyridoxamine (PM) that is the most potent agent of the three forms.
Pyridoxamine prevents the formation of AGEs. It works by trapping reactive carbonyl groups[9] and it also demonstrates free radical quenching properties.[10] Accumulation of AGEs is a physiologic consequence of tissue aging. Tissue deposits of AGEs and circulating AGEs are a hallmark of diabetes mellitus. Damage from AGEs is also seen in a variety of other vascular and degenerative diseases. AGE generation potentiates oxidative damage and lipid peroxidation in target tissues. The tissue damage caused by AGEs and ALEs further drives inflammation. The more we understand about the role of AGEs in ‘normal’ global aging, the more important a pharmacologic agent like pyridoxamine becomes.[11] Pyridoxamine has multiple mechanisms of action which can are best summarized in a study published in 2005. “…PM inhibits post Amadori steps of the Maillard reaction by sequestering catalytic metal ions and blocking oxidative degradation on Amadori intermediate.
PM also has the capacity to scavenge toxic carbonyl products of sugar and lipid degradation, and to inhibit reactive oxygen species.”[12] Pyridoxamine has been shown to limit the formation of AGEs without affecting glycemic control. There is additional evidence that high blood lipids contribute to the formation of AGE/ALEs even in the absence of hyperglycemia. Pyridoxamine inhibited the formation of both toxic end products demonstrating a protective effect on vascular and renal function in an animal model.[13] It is quite unique that PM demonstrates the ability to prevent lipid peroxidation and therefore ALE formation. Pyridoxamine inhibited the formation of ALEs by trapping malondialdehyde (MDA) an important intermediate in ALE formation.[14] The beauty of pyridoxamine lies not only in its multiple mechanisms of action, but also in its tolerability. There are little or no contraindications for its use.
The role of AGEs in health and disease
There are a variety of chronic and degenerative diseases associated with the accumulation of AGEs in tissues and organs. Many of these conditions we consider a normal part of growing older. The toxic AGEs can deposit anywhere compromising physiologic function. AGEs deposit in the lens of the eye leading to the formation of cataracts.[15] When AGEs deposit in the vascular system, long lived proteins such as collagen and elastin become stiff and hypertension is just one of many possible consequences.[16] It is hard to envision a chronic degenerative condition or disease of aging that would not benefit from the inhibition of AGE/ALEs formation. “Although AGEs in proteins are probably correlative, rather than causative, with respect to aging, they accumulate to high levels in tissues in age-related chronic diseases such as atherosclerosis, diabetes, arthritis and neurodegenerative disease.”[17] Inhibition of AGE formation could inhibit oxidative and inflammatory damage in target tissues, slowing the progression and pathophysiology of aging. This could lead to a significant improvement of the quality of life of the aging population. It is evident that AGE/`ALEs likely contribute to both diabetic and non diabetic vascular damage.[18] AGEs accumulate in tissues and organs in rheumatoid arthritis and Alzheimer disease.[19] As mentioned above, diabetes mellitus provides us with a model of accelerated aging. AGE/ALEs contribute to the various microvascular and macrovascular complications which occur in the disease.
Just as AGEs compromise the flexibility and efficiency in the vascular system, AGE deposition in the kidney compromise the kidney’s filtering capacity and can lead to nephropathy and kidney failure.[20] Culling the most recent literature was enlightening. There is now evidence that AGEs may contribute to colon cancer and melanoma.[21]
Pyridoxamine may prove to be a therapy for primary hyperoxaluria (which causes the formation of kidney stones.[22] AGEs may well interfere with osteoclast activity by altering the structural integrity of bone matrix proteins and osteoclast induced bone resorption, thereby contributing to an increased risk of bone fracture.[23] There may even prove to be a link between AGEs and osteoarthritis (OA) the most common cause of chronic pain and disability in older adults.[24]
In conclusion, pyridoxamine may prove one of our most powerful nutritional weapons in the war against chronic degenerative disease and aging itself.
[1] Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956; 2: 298-300
[2] Hipkiss AR. Accumulation of altered proteins and ageing: causes and effects. Exp Gerontol. 2006; 41(5):464-73
[3] Kikuchi S, Shinpo K, Takeuchi M, et al. Glycation-a sweet tempter for neuronal death. Brain Res Rev. 2003;41(2-3):306-23
[4] Ulrich P, Cerami A. Protein glycation, diabetes and aging. Recent Prog Horm Res. 2001;56:1-21
[5] Brownlee M. The pathological implications of protein glycation. Clin Invest Med. 1995; 18:275-82
[6] Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223-34
[7] Vlassara H. Advanced glycation in health and disease: role of modern environment. Ann N Y Acad Sci. 2005;1043:452-60
[8] Leklem, JE: Vitamin B6 In Handbook of Vitamins. 2nd Ed. Edited by L.J. Machlin. New York, Marcel Decker, 1991, 341-392
[9] Voziyan PE et al. A post Amadori inhibitor pyridoxamine. J Biol Chem. 2002;277(5):3397-3403
[10] Jain SK, Lim G. Pyridoxin and Pyridoxamine inhibit superoxide radicals and prevent lipid peroxidation and protein glycosylation. Free Rad Biol Med. 2001;30(3):232-37
[11] Negre-Salvayre A, Coattieux C, Inqueneau C, et al. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for inhibitors. Br J Pharmacol. 2007 [Epub ahead of print]
[12] Voziyan PA, Hudson BG. Pyridoxamine: the many virtues of maillard reaction inhibitor. Ann N Y Acad Sci. 2005;1043:807-16
[13] Alderson NL, Chachich ME, Youssef NN, et al. The AGE inhibitor of pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. Kidney Int. 2003; 63(6):2123-33
[14] Kang Z, Li H, Li G, Yin D. Reaction of pyridoxamine with malondialdehyde: mechanism of formation of advanced lipoxidation end- products. Amino Acids. 2006;30(1)55-61
[15] Padival S, Nagaraj RH. Pyridoxamine inhibits maillard reactions in diabetic rat lenses. Opthalmic Res. 2006; 38(5):294-302
[16] Bakris GL, Bank AJ, Kass DA, et al. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. Am J Hypertens. 2004; 17(12 Pt 2):23S-30S
[17] Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001;36(9):1527-37
[18] Mene P, Festuccia F, Pugliese F. Clinical potential of advanced glycation end product inhibitors in diabetes mellitus. Am J Cardiovasc Drugs. 2003;3(5):315-20
[19] Boulanger E, Puisieux F, Gaxatte C, Wautier JL. Aging: role and control of glycation. Rev Med Interne. 2007; [Epub ahead of print]
[20] Williams ME, Tuttle KR. The next generation of diabetic nephropathy therapies: an update. Adv Chronic Kidney Dis. 2005;12(2):212-22
[21] Yamagishi S, Nakamura K, Inoue H, Kikuchi S, et al. Possible participation of advanced glycation end products in the pathogenesis of colorectal cancer in diabetic patients. Med Hypotheses. 2005;64(6):1208-10
[22] Chetyrkin SV, Kim D, Belmont JM, et al. Pyridoxamine lowers kidney crystals in experimental hyperoxaluria: a potential therapy for primary hyperoxaluria. Kidney Int. 2005;67(1):53-60
[23] Valcourt U, Merle B, Gineyts E, et al. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and diffentiation. J Biol Chem. 2007; 28(8):5691-703
[24] Loeser RF Jr. Aging cartilage and osteoarthritis—what’s the link? Sci Aging Knowledge Environ. 2004;2004(29):p31